The last and most rigorous nineteenth-century confirmation of Avogadro's hypothesis arrived finally with the kinetic theory of gases and with the electromagnetism particularly with Boltzmann and Maxwell: the universal constant of gasses R and Faraday's one are directly proportional to Avogadro's number, and especially they turn out to be macroscopic expressions of the Boltzmann constant and of the electron charge respectively, microscopic fundamental quantity.
With the name of Avogadro's number it was proposed from Perrin to call the number of molecules contained in one gram-molecular weight (nowadays mole) of every substance, in her turn defined as the quantity of any substance containing in the beginning the same number of particles (the Avogadro's number) of that ones contained in 2 grams of molecular hydrogen, and from 1971 the same number of particles of that ones contained in 0.012 kg (12 grams) of 12C.
AAMOF Avogadro never directly face the problem to set absolutely the number of particles contained in a volume of gas (at fixed temperature and pression), since he was interested as chemist in establishing quantities of substances which could completely (without residues) react and in foretelling the quantity of products: he was named after the constant in order to recognize its direct derivation from the law put forward by him.
PROCEDURE:
The experiment's key point is the realization of a monomolecular (or monoatomic) substance's layer with known volume (with consequent low coefficient of compressibility), the calculation of its thickness and the use of the last to achieve the substance's molecular volume.
Without resorting to intricate electrochemical methods able to put down an all but monoatomic metal's layer, hard to manipulate though, we'll exploit the Archimedes's buoyancy acting on a little portion of a low-volatility fluid that makes it afloat on another one more dense, but primarily the strong intermolecular forces.
If the first one assure the monomolecularty of the thickness, making the molecules on deeper layers surface up to the contact surface with the air (lower-dense fluid than the considered one), the second one prevent the internal fragmentation of the layer that makes faster and more accurate the area's estimate.
| The (cis-)oleic acid meet our requirements: it has low surface tension (it expand quickly), high melting point, boiling temperature, vapour tension, and then generally low molecular kinetic energy, it has low density and even lower solubility in water because it' apolar, it's formed by very big molecules and furthermore it's very easy to trace, being the principal component of the olive oil (this was the liquid in the beginning used in the experiment, but as it is a medley, it varies its properties following the components' concentration) . |
| To increase the probability that the formed layer is monomolecular it's advisable to mix slowly the yellow oleic acid with hexane (not pentane since it has a too-high evaporation speed and it'd distort the concentration), an(other) apolar liquid, in a 1:10000-volume concentration- solution; in the lab-test initially it's been titrated a first solution, in a graded cylinder with 1ml of sensitivity, constituted by 1ml of oleic acid in 1 dl of hexane, then it's been titrated the final one in the same place diluting still further 1ml of the first solution in 1 dl of hexane. |
We transfered afterwards, through a pipette with 0.1cc of sensibility, 1ml of the second solution on the surface previously strewn with hydrophobic lycopodium powder to point out the otherwise invisible spot (use a salt cellar to avoid that it precipitate or get wet) of a tank (under which it'd been placed some graph paper) filled up with tap (not distilled) water. The solution's laying down in the tank should be executed veeeery slowly so as not to make the spot neither incorporate nor wet some lycopodium and in such a way as to be touched tank's rims: in either event the area turns out to be distorted since the oleic acid's spread is confined. In another utopia it would be advisable to obtain a round area, which has a small error of measurement. After a some minutes wait in which roughly all the hexane should have been vaporized, we snapped a photo on which we gauged the mark's area, and in which it appears clearly that the conditions above-mentioned have been by a long chalk remote from those ones realized in lab; nevertheless we have been at least able not to allow the contact spot-edge (we exploited a graphics drafting program to blacken the dry lycopodium).
INDIRECT MEASUREMENT:
Bearing in mind that the spot's volume now should be 1/10000 ml (it remains only the oleic acid), and gettig its surface graphically, we can start to estimate the layer's thickness, that is oleic acid molecule's one.
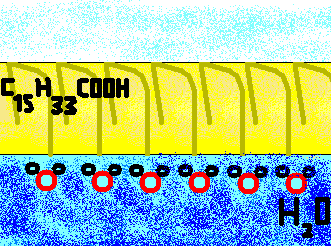 |
The oleic acid is formed by anfipathic molecules: the presence of the carboxyl group (COOH) always hydrophilic that lies on the C1C9 arm's tip allows us to understand that the molecule arranges itself with this extremity toward the water, with which it sets hydrogen bonds. The hydrophobic end is instead constituted by C10C13 arm, in which CH3 radicals prevail, that arranges itself toward the contact surface with the air: that's what is in outline showed by the picture on the left. | |
| But considering that the C1C9 arm basically is nothing but a chain of carbon-carbon bonds where it is hybridized (the hybridization of an atom is inferable from the the number of atoms that are bound to it: sp-->2, sp2-->3, sp3-->4) sp3 (except the C8 and the C9 that are hybridized sp2), we have considered each carbon from the 1 to the 8 centre of a tetrahedron as in the picture. At this point we can suppose roughly the molecule's longer arm C1C9 to be its thickness without the gap which separate it between the water molecules beneath, namely the hydrogen bonds' distance. | ||
Then the length of the whole arm will turn out to be 4 times the distance between C1 and C3, that is tied up with the C1 and C2's one as the tetrahedron's side is tied with the radius of the circumscribing sphere. (the two pictures with the white background don't portray the real molecule's shape: they have been built to illustrate the different arms).
| However, not knowing exactly number and spatial disposition of these hydrogen bonds, nothing remains for us but to be content with testing the similarity of the orders of magnitude. When this was established, we'd proceed with the calculus with the same method (associating it honestly a relative error very high) of the length of the arm C10C13, that according to arguments similar to previous ones, should position itself around the three carbon-carbon single bonds. Being acquainted with the real molecule's form (in the paragraph above "procedure"), seeing that the arm C14C18 is slightly inclined respect to the plane that contains the other two ones, we approximated the molecule to an elliptical base cylinder where the arm C1C3 represents the ellipse's diagonal, with lenght comprised between that one of the shorter axis and that one of the longer one, in order to appraise its volume. |
| Passing by the single molecule's volume we could then proceed to the calculus of its mass, beeing known for us the oleic acid density, and from here finally to the Avogadro's constant, ratio between the molar mass of the oleic acid and the mass of its molecule. |