Execution of the experiment
The most elementar way to valuate Avogadro's number is trying to obtain a layer of matter so thin thath we can suppose it's unimolecular (made only by one molecule).
There are many ways to obtain sucha a thin layer of matter. The most intuitive is to reduce in sheets a malleable metal. However, using this method you can't be sure the sheet is really unimolecular, and obtaining such a thin sheet is very difficult.
Another way is to drop a little quantity of oil on a liquid such as water. Oil molecules are much bigger than water ones, so oil tends to remain on the surface and to expand all over the recipient, until the spot is unimolecular in thickness or it reaches the limts of the recipient.
If the volume of the oil dropped and the area of the spot on the water surface are known, it is possible to make an evaluation of the molecule's thickness, which is equal to the ratio between the volume and the area of the spot.
At this point, to evaluate more of the molecule's charateristics it is necessary to make an hypothesis about its shape. For instance, the molecule could be a cube, a sphere, or a cylinder.
If the shape of a molecule is known (and then it is possible to calculate its volume), it becomes possible to calculate the number of molecules in the spot (which is equal to the ratio between the volume of the spot and the volume of a molecule) and the mass of one molecule (which is equal to the volume of a molecule multiplied by its density).
If the mass of 1 mole of oil and the mass of a molecule of oil are known, it is possibleto make an evaluation of Avogadro's number, which is the ratio between the two masses.
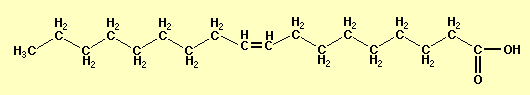
The oil that has been used in our experiment is the oleic acid (the main component of olive oil), which has chemical formula C18H34O2.

The oleic acid has a density smaller than water has (ρacido oleico = (8,73 ± 0,01) · 10-1 g/cm 3) and therefor it can't be mixed with it. Besides, oleic acid has a small superficial tension.
Oleic acid has also another property: its molecules are very long and composed of two parts. One of them is apolar and hydrophobic (it rejects water), the other one is polar and hydrophilic (it's attracted by water). This is because the hydrophilic part can establish hydrogen bonds with water, while the other part can't and it's electrically rejected. Because of this property, oleic acid molecules tend to dispose vertically on water. This is an important factor in evaluating Avogadro's number.
If too much oleic acid is used to create the spot, it can cover all water's surface, making it impossible to verify if the spot is really unimolecular. To avoid this danger, in the experiment oleic acid is used mixed with hexane, a volatile solvent, in a 1/10000 solution.
To make it evident the spot of oleic acid (transparent) on water (also transparent) it was used lycopodium, a fossil powder. It was spread all over the surface of water before the experiment.
Lycopodium was then "pushed away" by oleic acid, making the shape of the spot more evident.
| To the previous page | To the next page |