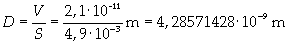
Data elaboration - First spot
If the volume of the oil spot and its area, it is possible to give an evaluation of thickness D.
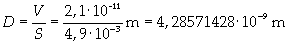
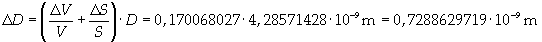
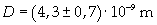
Supposing the spot has unimolecular thickness, it is possible to think that thickness D is the thickness of the molecule.
In this experiment there is no experimental information about the shape of the molecule, so it is necessary to use a hypotetical model of its shape. For instance, we can hypotize the molecule is a cube or a cylinder.
Cube-shaped molecule
Supposing the molecule is a cube, the volume of one molecule ("molecola" in Italian) is easily calculated, taking D as its edge length:
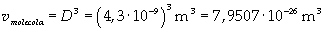
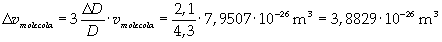
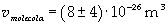
Now it is possible to give an evaluation of number n of molecules contained in the spot of oleic acid, as ratio between the volume of the spot itself and the volume of one single molecule:
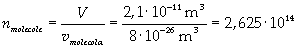
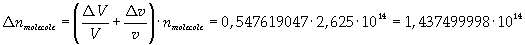
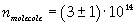
The density of oleic acid is known, so it is possible to calculate mass m of a singol molecule:
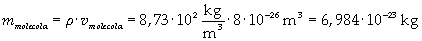
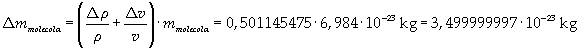
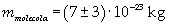
The mass of 1 mole of oleic acid (0,2825 kg) is known, too, so it is possible to evaluate Avogadro's number N:
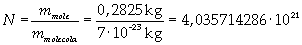
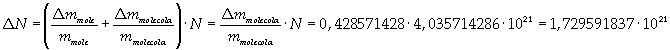
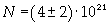
Avogadro's number evaluated using a cubic model for the oleic acid molecule is one hundred times smaller than real Avogadro's number (6,022 · 10 23).
Evaluation has a relative error of 1/2 = 0,5.
Sphere-shaped molecule
Supposing the molecule is a sphere, the volume of one molecule is easily calculated, taking D as its edge length:
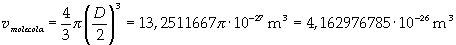
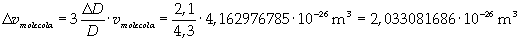
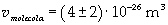
Now it is possible to give an evaluation of number n of molecules contained in the spot of oleic acid, as ratio between the volume of the spot itself and the volume of one single molecule:
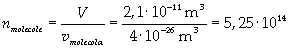
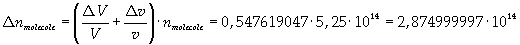
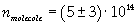
The density of oleic acid is known, so it is possible to calculate mass m of a singol molecule:
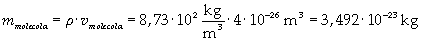
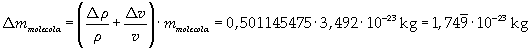
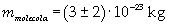
The mass of 1 mole of oleic acid (0,2825 kg) is known, too, so it is possible to evaluate Avogadro's number N:
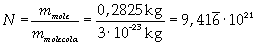
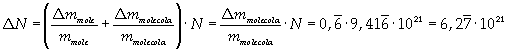
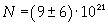
Avogadro's number evaluated using a cubic model for the oleic acid molecule is one hundred times smaller than real Avogadro's number (6,022 · 10 23).
Evaluation has a relative error of 2/3 = about 0,67.
Real oleic acid molecules are very long and tend to dispose vertically on water (as described in another chapter).
Because of this, a "longer" molecule model could lead to more realistic results. So, we could use a molecule shaped as a cylinder, with base diameter equal to 1/2,1/4 or 1/8 of the height.
Cylinder-shaped molecule with base diameter equal to one half of the height

Supposing the molecules are cylinder-shaped, the radium of a single molecule can be calculated taking thickness D as height of the molecule.
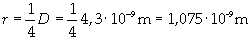
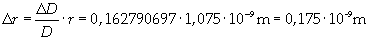
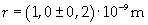
The volume is:
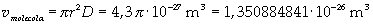
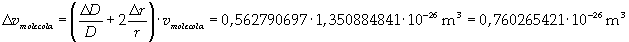
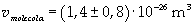
Now it is possible to give an evaluation of number n of molecules contained in the spot of oleic acid, as ratio between the volume of the spot itself and the volume of one single molecule:
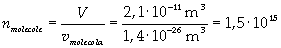
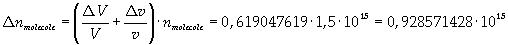
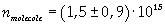
The density of oleic acid is known, so it is possible to calculate mass m of a singol molecule:
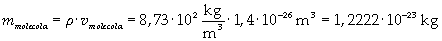
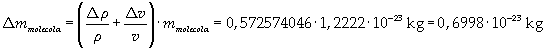
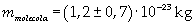
The mass of 1 mole of oleic acid (0,2825 kg) is known, too, so it is possible to evaluate Avogadro's number N:
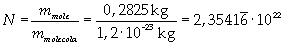
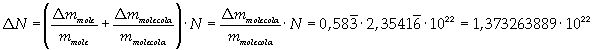
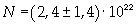
Avogadro's number evaluated using a cubic model for the oleic acid molecule is one hundred times smaller than real Avogadro's number (6,022 · 10 23).
Evaluation has a relative error of 7/12 = about 0,58.
Cylinder-shaped molecule with base diameter equal to one quarter of the height

Supposing the molecules are cylinder-shaped, the radium of a single molecule can be calculated taking thickness D as height of the molecule.
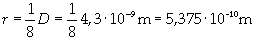
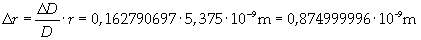
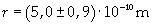
The volume is:
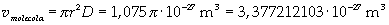
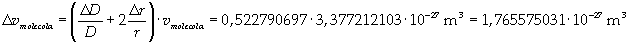
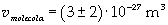
Now it is possible to give an evaluation of number n of molecules contained in the spot of oleic acid, as ratio between the volume of the spot itself and the volume of one single molecule:
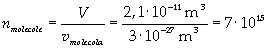
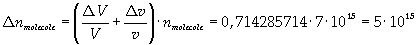
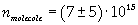
The density of oleic acid is known, so it is possible to calculate mass m of a singol molecule:
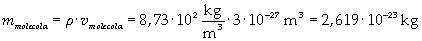
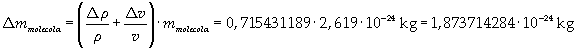
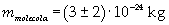
The mass of 1 mole of oleic acid (0,2825 kg) is known, too, so it is possible to evaluate Avogadro's number N:
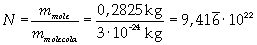
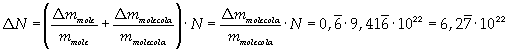
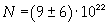
Avogadro's number evaluated using a cubic model for the oleic acid molecule is one hundred times smaller than real Avogadro's number (6,022 · 10 23).
Evaluation has a relative error of 2/3 = about 0,67.
Cylinder-shaped molecule with base diameter equal to one eighth of the height

Supposing the molecules are cylinder-shaped, the radium of a single molecule can be calculated taking thickness D as height of the molecule.
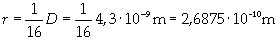
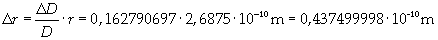
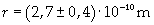
The volume is:
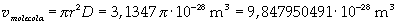
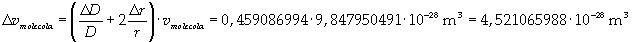
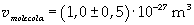
Now it is possible to give an evaluation of number n of molecules contained in the spot of oleic acid, as ratio between the volume of the spot itself and the volume of one single molecule:
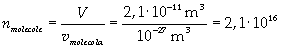
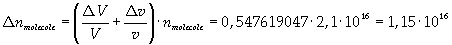
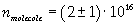
The density of oleic acid is known, so it is possible to calculate mass m of a singol molecule:
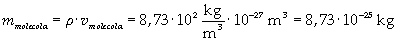
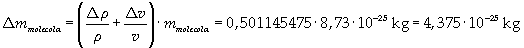
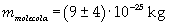
The mass of 1 mole of oleic acid (0,2825 kg) is known, too, so it is possible to evaluate Avogadro's number N:
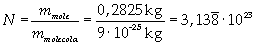
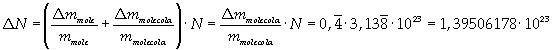
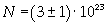
Avogadro's number evaluated using a cubic model for the oleic acid molecule is one hundred times smaller than real Avogadro's number (6,022 · 10 23).
Evaluation has a relative error of 1/3 = about 0,33.
| To the previous page | To the next page |